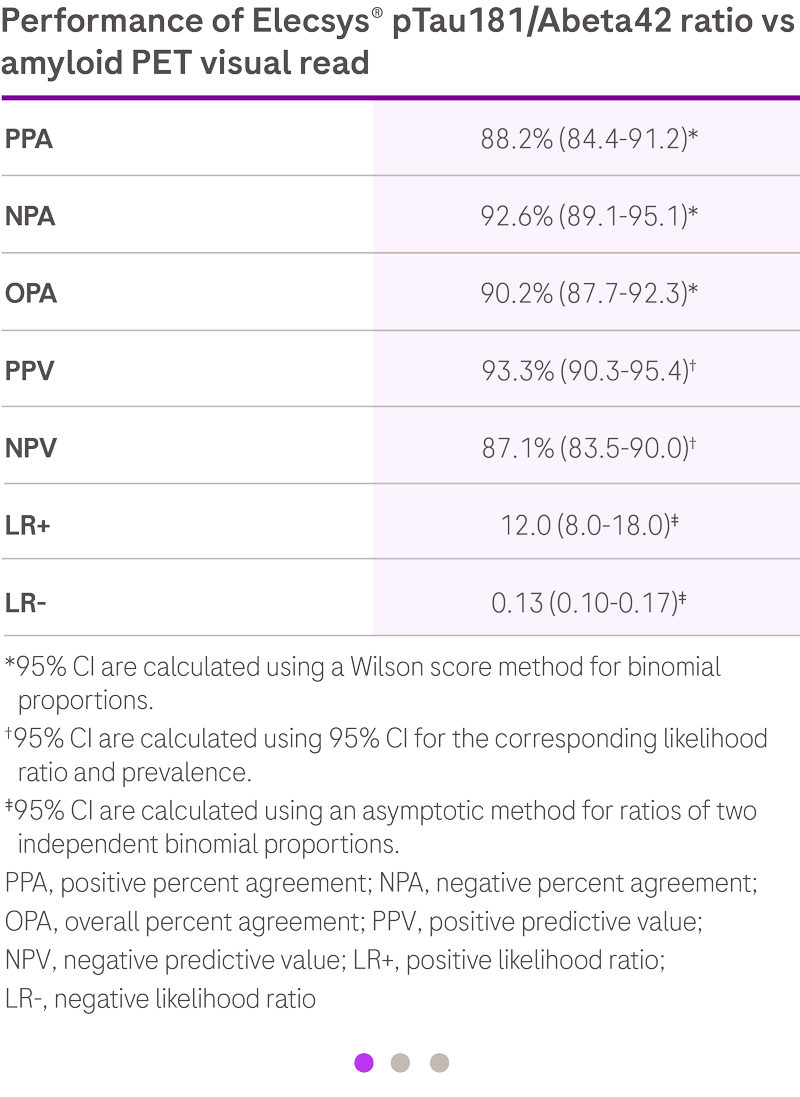
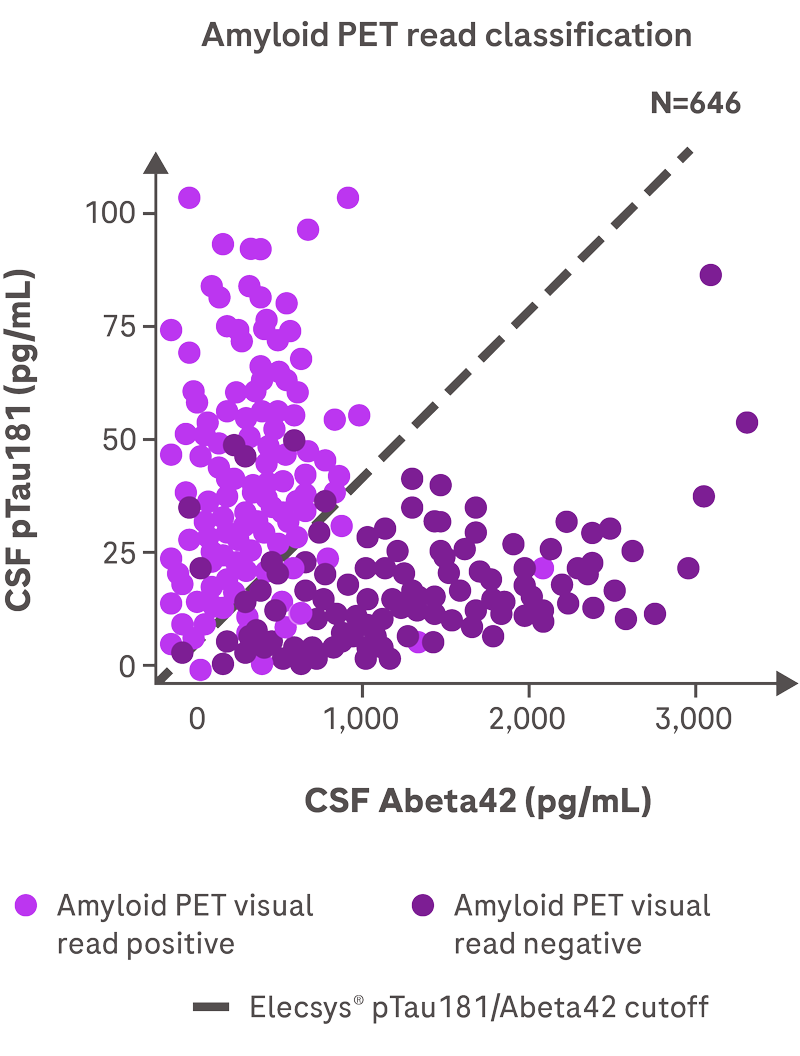
A negative result, defined as pTau181/Abeta42 ratio value below cutoff or an Abeta42 value
above the measuring range, is consistent with a negative amyloid positron emission
tomography (PET) scan result. A negative result reduces the likelihood that a patient's
cognitive impairment is due to AD. A positive result, defined as pTau181/Abeta42 ratio value
above cut-off, is consistent with a positive amyloid PET scan result. A positive result
does not establish a diagnosis of AD or other cognitive disorder.
The pTau181/Abeta42 ratio result is used as an adjunct to other clinical
diagnostic evaluations.1
Elecsys® AD CSF ratio achieves 90% concordance with amyloid PET1,2