In the United States, over 6.9 million people live with Alzheimer’s disease (AD), and the number of cases is expected to rise to 13 million by 2050. In fact, one in nine Americans over 65 have Alzheimer’s dementia, the most common form of dementia. In total, the disease has an estimated economic cost on the U.S. economy of $360B.2
After decades waiting for impactful treatments, there’s finally hope. With recent healthcare advancements, having an accurate, confirmed diagnosis early in the disease will enable those affected by AD to take advantage of new therapies and also to make necessary changes to their lifestyle to help delay onset of dementia and/or disease progression. As a result, an accurate, confirmed diagnosis is more important than ever.
Cerebrospinal fluid biomarkers support early diagnosis of Alzheimer’s
Timely diagnosis of Alzheimer’s disease is an unmet need in clinical practice. The diagnosis of Alzheimer’s is often one of exclusion, determined by ruling out non-AD causes of symptoms through several evaluations such as cognitive exams, laboratory tests and neuroimaging. Unfortunately, this process not only could delay the formal diagnosis, but it was shown that clinical diagnosis can be inaccurate in approximately 23% of cases.2,3 The use of cerebrospinal fluid (CSF) biomarkers could positively impact clinical diagnosis for Alzheimer’s disease.
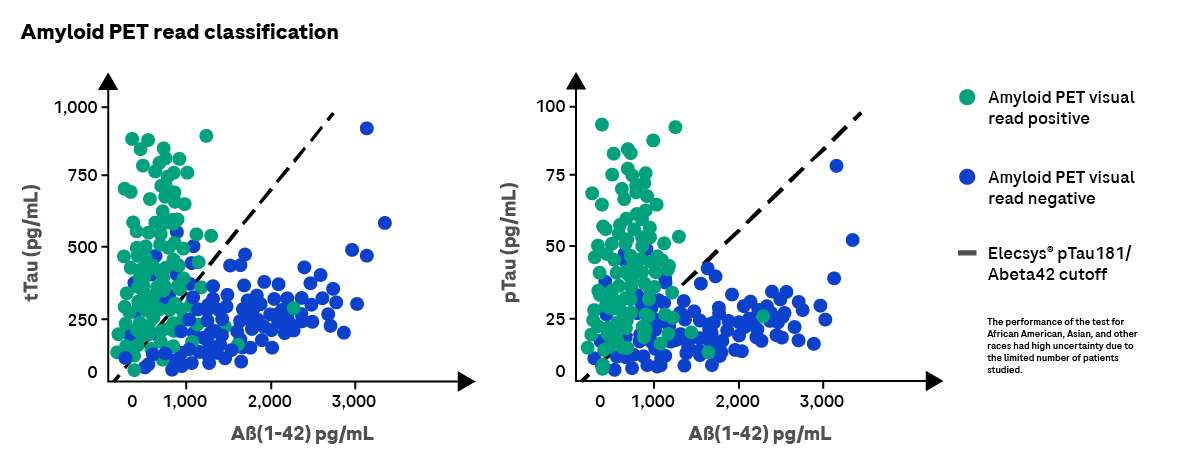
In addition to other clinical investigations, an early Alzheimer’s diagnosis can be supported by CSF biomarker tests that measure beta-amyloid protein (1-42) (Abeta42), phosphorylated tau protein (pTau) and total Tau protein (tTau). Changes in these biomarkers reflect the specific Alzheimer’s pathologies such as the accumulation of abnormal amyloid-beta and tau in plaques, in neurofibrillary tangles, which are followed by neurodegeneration.
Our FDA-cleared Elecsys® Alzheimer’s disease CSF biomarker portfolio can aid in the confirmed diagnosis of Alzheimer’s disease for those with mild cognitive impairment (MCI) and mild Alzheimer’s disease dementia, and can support treatment decisions for patients presenting in these early disease stages. Learn more.
Early diagnosis can benefit patients and society
An early Alzheimer’s disease diagnosis can lead to patient eligibility for new therapies targeted at early stages of the disease. In addition, it allows patients time to take preventative measures to safeguard their cognitive functions, prolong their ability to live independently and enhance their quality of life. By facilitating access to treatment and management of the disease in early stages, a timely diagnosis could potentially significantly lower the medical and long-term care costs for this disease.2 Learn more.