



*Ten cobas Liat SARS-CoV-2 kits (200 individual tests) + one SARS-CoV-2 control kit OR *Eight cobas Liat SARS-CoV-2\Flu kits (160 individual tests) + one SARS-CoV-2 Flu control kit
It's easy to “Go PCR” wherever patient care is needed with our special offer.
Simply scan the QR code or tap the link to begin.
The cobas Liat PCR System: Delivering the greatest confidence in patient care
Our system provides accurate, actionable results using multiplex and single assays to identify the correct pathogen:
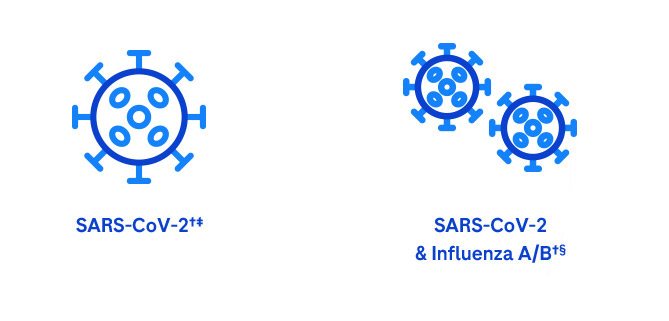
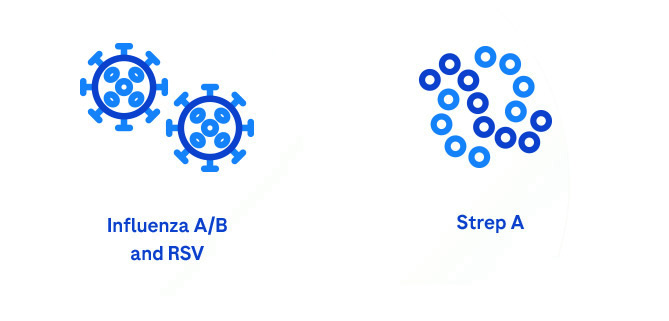
Simplify PCR testing wherever care is needed.

One minute
of hands-on time 1
20 minutes or less
to get the results you and your patients need

* cobas SARS-CoV-2 & Influenza A/B assay: If co-infection with influenza A or influenza B virus is suspected in samples with a positive SARS-CoV-2 result, the sample should be re-tested with another FDA cleared, approved, or authorized influenza test, if influenza virus detection would change clinical management.
+ This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. S 360bbb3bX1), unless the declaration is terminated or authorization is revoked sooner.
+ This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
This test has been authorized only for the simultaneous qualitative detection and differentiation of nucleic acid from SARS-CoV-2, influenza A virus, and influenza B virus, and not for any other viruses or pathogens.
Reference:
1. Nolte FS, et al. J Clin Microbiol. 2016;54(11):2763-2766. doi:10.1128/JCM.01586-16

SEND ME INFORMATION
Diagnostics systems using gold-standard PCR technology

LET'S CONNECT
Connect with a Roche representative about PCR solutions for your office