Investing today in tomorrow’s healthier communities
By building strong partnerships with labs, Roche provides high performance instruments, time-saving automation, trusted expertise, and unparalleled service and support. Our dedication to research and development enables clinicians and labs to provide patients with innovative, dependable sexual health testing and care. Roche’s solutions in molecular testing and sexual health enable better patient outcomes for everyone, in every community.
Roche STI Portfolio
cobas® CT/NG
cobas® CT/NG provides a solution to meet the growing demand for CT/NG Testing, delivering exceptional assay performance, providing broader information for improved patient care decisions, and enabling simplicity and flexibility to support varying throughput and workflow requirements.
- The Dual-Target approach of cobas® CT/NG of both the genomic and cryptic plasmid DNA of Chlamydia trachomatis, ensures inclusion of nvCT strains for a highly sensitive test.
cobas® CT/NG has been validated for use with male and female urine, clinician instructed self-collected vaginal swab specimens (collected in a clinical setting), and clinician-collected vaginal swab, endocervical swab, oropharyngeal swab and anorectal specimens collected in cobas PCR media and cervical specimens collected in PreservCyt Solution. This test is intended as an aid in the diagnosis of chlamydial and gonococcal disease in both symptomatic and asymptomatic individuals.
cobas® TV/MG
cobas® TV/MG provides an efficient solution to meet the growing demand for STI testing by combining two key targets into one assay. With demonstrated assay performance across a broad set of specimen types, cobas® TV/MG provides an easy and convenient method for reliable STI testing.
- Highly sensitive test using a multi-copy exclusive target for TV and dual-target design for MG
- Exceptional performance demonstrated in urogenital samples
- Validated for IVD use for TV testing both male and female patients
cobas® TV/MG has been validated for use with female urogenital specimens, including urine, clinician-collected and clinician-instructed self-collected vaginal swab and endocervical swab specimens all collected in cobas® PCR media – and cervical specimens* collected in PreservCyt® Solution. Also validated for use with male urine and clinician-collected and clinician-instructed self-collected meatal swab** specimens.
* TV only
** MG only
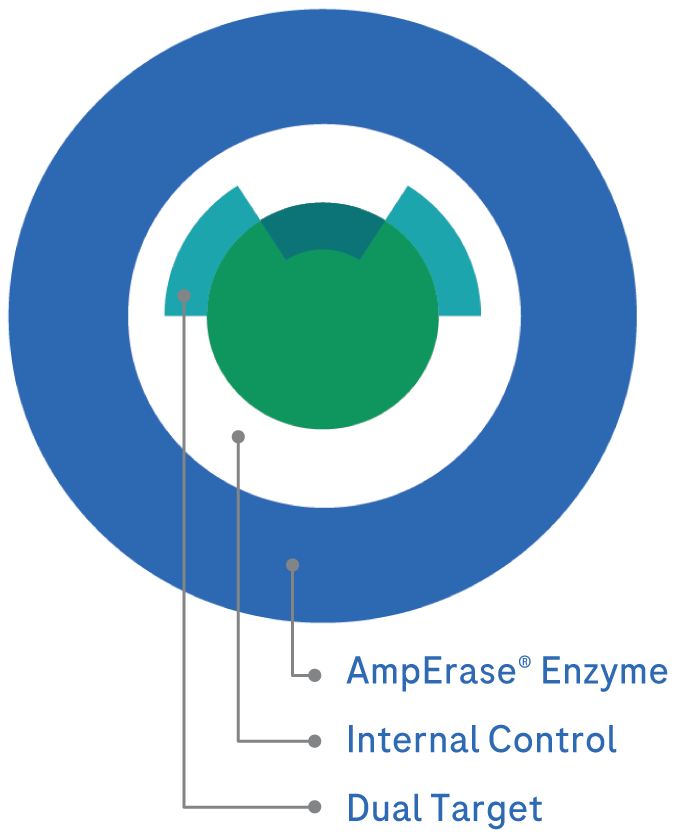
Protect the integrity of lab results with layers of protection
Dual targets for CT, NG, MG, multi-copy exclusive target for TV, an Internal Control and the AmpErase® enzyme with fully automated contamination control built into every assay, which adds up to powerful productivity with confidence in test results.
Additional testing available:
- cobas® HPV
- cobas® HPV self-collection
- cobas® HIV-1
- cobas® HIV-1/2 Qual
- cobas® HBV
- cobas® HCV
- cobas® HBV RNA (RUO) ‡
- cobas® HDV (RUO) ‡
- NG Resistance (RUO) ‡
Testing in development:
- cobas® HPV Next Generation genotyping beyond HPV 16 & 18) †
- cobas® HPV home sampling claim extension †
- cobas® STI home sampling claim extension †
- cobas® Lesion panel (HSV 1/2, VZV & syphilis) †
- cobas® BV / CV †
- MG Resistance (RUO) ‡ †
† This product is in development. This product is not available for use in the U.S.
‡ For Research Use Only. Not for use in diagnostic procedures.
Now introducing Roche HPV Self-Collection Solution
The cobas® HPV assay has recently gained FDA approval for use with self-collected vaginal specimen in a healthcare setting. Women and people with a cervix can collect their own vaginal specimen in a private healthcare setting when a cervical specimen can not be obtained.
This solution
- Reduces barriers to testing and increases accessibility
- Empowers and enables women to better manage their own healthcare
- Improves cervical cancer screening attendance. *
Learn more: Expanding access to cervical cancer screening with HPV self-collection →
* Elfström KM, et al. Increasing participation in cervical screening by targeting long-term nonattenders: Randomised health services study. Int J Cancer. 2019 Dec.
Workflow
Run up to 3 tests simultaneously for faster turnaround
Simultaneous testing of infectious disease, respiratory, sexual health, and transplant assays leads to dramatic time savings and increased efficiency.
Three assays run simultaneously
Continuous loading of samples with no batching or pre-sorting required for mixed test requests
Efficient workflow
Full automation and process control of all STI tests onto a single platform, including LDTs
Single sample
Simultaneous processing of multiple tests from the same patient sample
Universal Instruments
Maximize throughput without adding team members or resources
Empowering laboratories to balance productivity, efficiency, and potential cost effectiveness with fully automated and standardized workflows, on demand testing and the proven reliability of Roche quality results.
Proven Performance
Generate trusted, reproducible results with proven performance
Absolute Automation
Leverage absolute automation to drive increased efficiency
Unmatched Flexibility
Deliver unmatched flexibility to meet your molecular testing demands – today and tomorrow